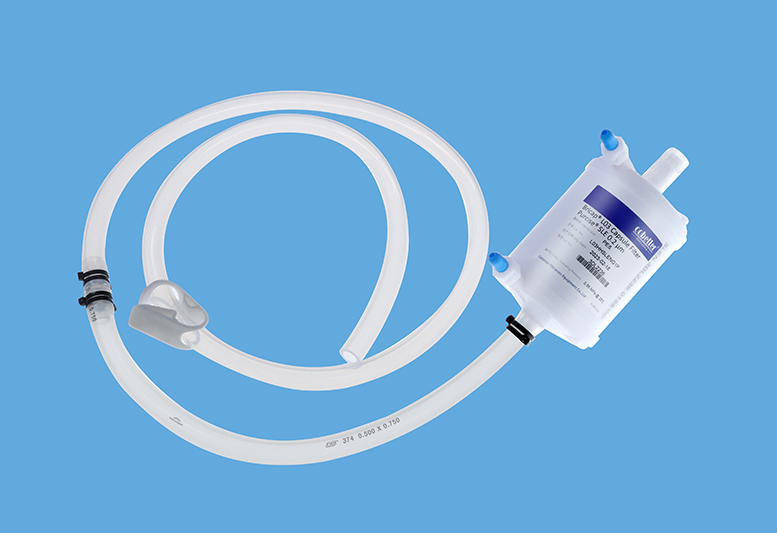
Lifemeta™ STF TPE チューブ
Cobetter Lifemeta™ STF TPEチューブは、医療グレードの熱可塑性エラストマー(TPE)材料から押し出された高性能フレキシブルチューブで、優れたヒートシール性と滅菌溶着性能を備えています。内壁は滑らかで剥離が少ないです。Lifemeta™ STFは、市場の主要なTPEチューブと比較して、かなりの溶接互換性と溶着性を実証しています。従来のシリコンチューブや PVC チューブと比較して、この製品は化学的適合性が広く、さまざまな作業条件下で優れた物理化学的性能を維持できます。
推奨の業界アプリケーション:
お見積りのお問い合わせ
概要
特徴
- 滅菌溶着およびヒートシール
- 優れた低吸収性と低結合性
- シリコン樹脂に比べて透過性が低い
- 滑らかな内壁、優れた流動性
- 抜群の引張強度
アプリケーション
- 細胞培養培地および緩衝液の調製
- 液体サンプリング システム
- ろ過プロセス
- 滅菌充填
- 滅菌切断および接続
- シングルユースチューブ アセンブリ
製品性能
| Tubing Series | Lifemeta™ STF |
| Material | TPE |
| Durometer Hardness (Shore A) | 60±5 |
| Appearance | Translucent |
| Aseptic Sealing and Welding | √ |
| Peristaltic Pump Life | Limited |
| Min. Temperature | -58℉ | -50℃ |
| Max. Temperature | 284℉ | 140 ℃ |
| Gamma Stability (max. 50 kGy) | √ |
| Sterilization Methods | Autoclave Gamma irradiation |
| Validation Guide | √ |
| Packaging | Double layer PE bag packaging |
| Recommended usage | Culture media and buffers preparation Fermentation/Cultivation Purification Aseptic Sealing and Welding |
Regulatory Compliance
| Bacterial Endotoxin | Aqueous extraction contains < 0.25 EU/mL as determined by Amebocyte Lysate, USP <85>. |
| USP <87> Cytotoxicity | Meet the requirement of USP <87> In Vitro Biological Reactivity Test. |
| USP <88> Biological Reactivity | Meet the criteria of the USP <88> Biological Reactivity Test for Class VI plastics. |
| Animal Derivative Content | Products do not contain animal derived components and are free from TSE risk. |
| Quality Assurance | These products are manufactured in a facility which adheres to ISO 9001:2015 Practices. |
Sterilization Methods
| Gamma Irradiation | Can be gamma irradiated at 25-45 kGy in sterile package and can not be re-sterilized. |
| Autoclave | Can be autoclaved 25 cycles for 30 minutes at 121 °C. |
Physical characteristics
| Item | Value | Procedure |
| Durometer Hardness (Shore A) | 60 ± 5 | ASTM D2240 |
| Burst Strength | 0.4-0.6 MPa | ASTM D380 |
| Tensile Strength | 5.17-5.18 MPa | ASTM D412 |
| Elongation at Break | 742-776% | ASTM D412 |
| Vacuum Resistance | > 30 kPa | ISO 7233: 2016 |
| Temperature Range | -50-140 °C |
Note: Please refer to the validation guide document for detailed test methods and results.
ご注文情報
| Part Number | I.D. | O.D. | Wall Thickness | Tube Number | Packaging* | Qty(m/pk) | |||
| In. | mm. | In. | mm. | In. | mm. | ||||
| STFL150N | 1/8" | 3.2 | 1/4" | 6.4 | 1/16" | 1.6 | 16# | Non-sterile | 15 |
| STF25150N | 3/16" | 4.8 | 5/16" | 8 | 1/16" | 1.6 | 25# | Non-sterile | 15 |
| STF15150N | 3/16" | 4.8 | 3/8" | 9.6 | 3/32" | 2.4 | 15# | Non-sterile | 15 |
| STF17150N | 1/4" | 6.4 | 3/8" | 9.6 | 1/16" | 1.6 | 17# | Non-sterile | 15 |
| STFR150N | 1/4" | 6.4 | 7/16" | 11.2 | 3/32" | 2.4 | 24# | Non-sterile | 15 |
| STF26150N | 1/4" | 6.4 | 1/2" | 12.7 | 1/8" | 3.2 | 26# | Non-sterile | 15 |
| STF35150N | 5/16" | 8 | 1/2" | 12.7 | 3/32" | 2.4 | 35# | Non-sterile | 15 |
| STFY150N | 3/8" | 9.6 | 5/8" | 15.9 | 1/8" | 3.2 | 73# | Non-sterile | 15 |
| STFH150N | 1/2" | 12.7 | 3/4" | 19.1 | 1/8" | 3.2 | 82# | Non-sterile | 15 |
| STFN150N | 3/4" | 19.1 | 1" | 25.4 | 1/8" | 3.2 | 90# | Non-sterile | 15 |
| STFM100N | 3/4" | 19.1 | 1-1/8" | 28.6 | 3/16" | 4.8 | 191# | Non-sterile | 10 |
| STFD050N | 1" | 25.4 | 1-3/8" | 34.9 | 3/16" | 4.8 | 92# | Non-sterile | 5 |
Note: The table is the standard product number for sale, if you need special specifications (length, sterile packaging), please communicate the requirements in advance.
* Packaging: This product is available in sterile or non-sterile packaging. The sterilization method for sterile products is gamma irradiation, and the irradiation dose is 25~45 kGy. Naming of products with different packaging methods refer to the following:
1. Non-sterile: STFL150N
2. Sterile:STFL150S
 eShop
eShop